
Description
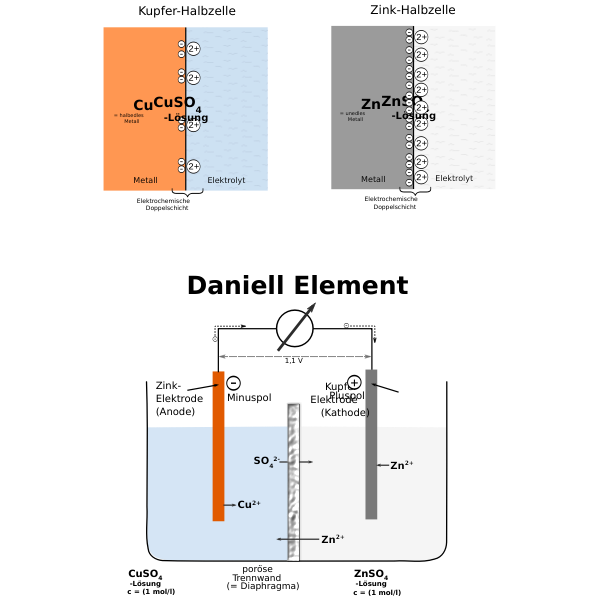
The Daniell cell is also the historical basis for the contemporary definition of the volt, which is the unit of electromotive force.In the Daniell cell, copper and zinc electrodes are immersed in a solution of copper(II) sulfate and zinc sulfate respectively. At the anode, zinc is oxidized.The Daniell cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consisted of a copper pot filled with a copper sulfate solution, in which was immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode.
SVG ID
108446
Size
0.13 MB
No. of downloads:
77
Date:
27/03/2020
License:
Public Domain
SVG published by: